China Focus: 3 years of fighting COVID-19 with vaccines, drugs

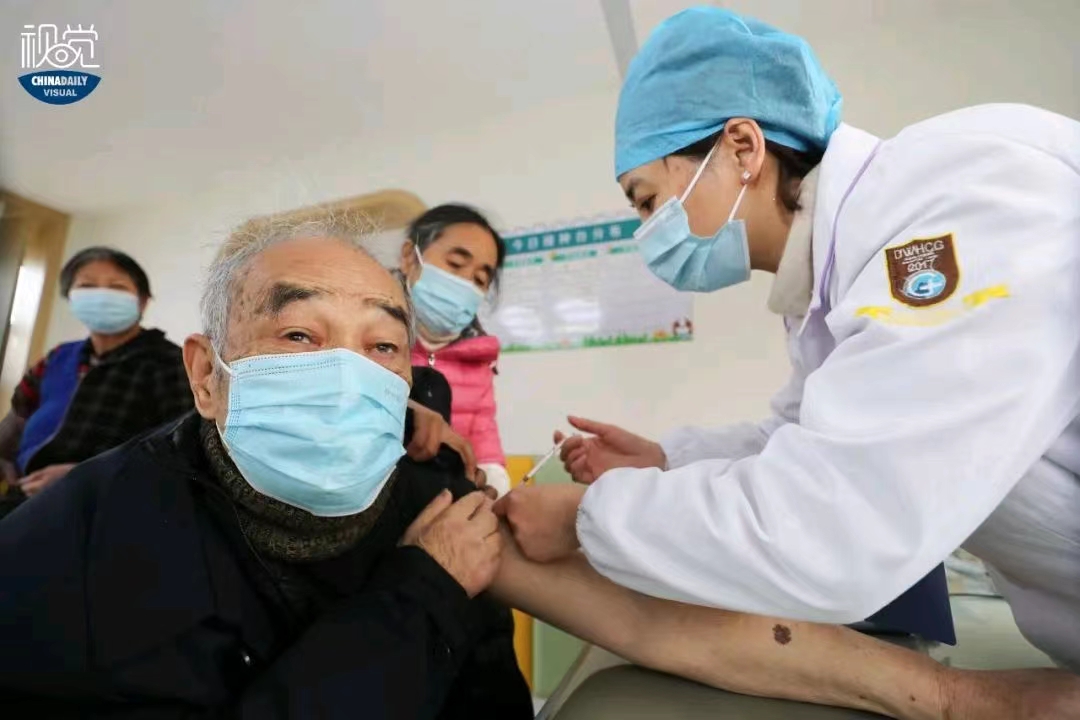
BEIJING -- China has withstood multiple COVID-19 waves in the past three years, aided by massive vaccination drives and self-developed medicines.
The country, with more than 1.4 billion people, has effectively coped with over 100 COVID-19 epidemic outbreaks within three years and managed to keep its incidence rate, severe illness rate and mortality rate at the world's lowest level.
The latest data has shown that the rate of COVID-19 full inoculation -- including a booster injection -- exceeded 90 percent in the whole population, forming an effective immune bulwark against the challenge posed by the rapidly-mutating virus.
About 3.45 billion vaccine jabs had been administered by Dec 7, 2022, and more than 228 million people above 60 had been given full inoculation by Nov. 28, accounting for over 86 percent of the total population in that age group, according to the National Health Commission.
After the first epidemic outbreak in 2019, China immediately shared the first completed genome sequence of the novel coronavirus and kicked off the research and development of vaccines.
In April of 2020, a Chinese adenovirus vector vaccine started the second phase of clinical trials, the first COVID-19 vaccine in the world that had entered that stage. Two COVID-19 inactivated vaccine candidates for clinical trial were also being developed. Now, all three of them have been included on the World Health Organization list of vaccines for emergency use.
China's vaccines have played a crucial role in global responses to the epidemic, as the country continuously offered vaccine aid to less developed countries.
As of Aug 23, China had provided 189 million doses of COVID-19 vaccines to 27 African countries. This vaccine support started after the Eighth Ministerial Conference of the Forum on China-Africa Cooperation in November 2021. Localized annual production capacities, in cooperation with African partners, reached nearly 400 million doses.
As the virus kept mutating in the past three years, so Chinese scientists continued stepping up their efforts to counter it.
By October this year, at least 46 COVID-19 vaccines were being tested in human trials in China, and more than 20 in overseas clinical trials, as the country pushed forward its vaccine research and development through multiple tech routes.
Among them, three inactivated monovalent vaccines designed to neutralize Omicron variants, are in testing for sequential immunization in the Chinese mainland, Hong Kong and the United Arab Emirates -- and those tests are going well.
Also, domestically-developed mRNA-based COVID-19 vaccine candidates and broad-spectrum ones are also in the pipeline in China.
The country is developing vaccines targeting easier drug-delivery as well. A vaccine administered via oral inhalation has been approved for emergency use among populations who have been inoculated with two doses of inactivated vaccines for a period of six months or longer.
Until now, 13 COVID-19 vaccines developed via different tech routes have gotten conditional market approval or been greenlighted for emergency use in China.
THERAPIES
Also, scientists and clinicians in China introduced a series of COVID-19 therapies that combined cutting-edge molecular research and traditional Chinese medicine, in order to effectively improve clinical treatment quality.
Five months after the initial outbreak of the novel coronavirus, a research team from the Chinese Academy of Sciences announced that JS016, a COVID-19-neutralizing antibody, could be administered to healthy people in clinical trials. And in November of 2021, the antibody was granted emergency-use authorization in 15 countries, including the United States and several European countries.
In December of 2021, a cocktail therapy of monoclonal antibodies BRII-196 and BRII-198 was approved for emergency use, and in July this year, China's drug regulator granted conditional authorization for its first homegrown oral COVID antiviral drug.
Now, China is developing anti-COVID drugs via three tech routes -- blocking the virus from making inroads into cells, inhibiting its replication and employing the human immune system. Some of these candidates have been advanced to the third stage of clinical trials.
With such efforts, the country's average life expectancy saw a steady growth despite the pandemic. It increased from 77.93 in 2020 to 78.2 in 2021.
- 102-year-old veteran recalls war, hails China's rise
- China enhancing childcare services with eye on fertility rate
- Monkeys thriving at a research base in Hubei
- Youth exchange fosters cross-cultural friendships
- China expels Japanese vessel for illegally entering waters
- Explore Tianjin: Is everyone here so optimistic?